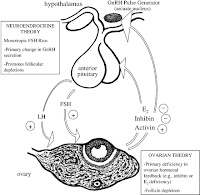
Figure 1. Aging and the female reproductive system. Reprinted from Figure 2 in Maturitas 30:193–204 by Soules MR, Battaglia DE, Klein NA; "Inhibin and reproductive aging in women," copyrighted 1998 by Elsevier Ireland Ltd. (22) Redrawn by Kimberly A. Ottinger.
Paper: Overlie, I., Morkrid, L., Anderson, A., Skakkebaek, N., Moen, M., and Holte, A. 2005. Inhibin A and B as markers of menopause: a fiver year prospective longitudinal study of hormonal changes during the menopausal transition. Acta Obstet Scand 2005: 84: 281-285. Available online March 26th 2007.
Summary: Serum Follicle stimulating hormone (FSH) is referred to as the endocrine marker of menopause. This is due to the high levels (5-10 times greater than that of a reproductive follicle age ) of FSH during the postmenopasual period. Menopause is defined after 12 months of amenorrhea following the final menstrual period (FMP). The purpose of this study was to find a more accurate marker of menopause. This was achieved by analysing inhibin A and B, FSH, LH and estradiol among 59 women without hormonal treatment during perimenopause (the period in a womens life in which her body begins its transition into menopause, it includes the years leading up to menopause — anywhere from two to eight years — plus the first year after your final period), and early postmenopause (the time when most of the transitional stress of menopause has passed.)
Random venous blood samples of fifty nine women ranging in age from 46-56 were taken and analyzed annually for five years during the menopausal transition. In the analysis the hormones inhibin A and B, FSH, LH and estradiol were examined. Inhibin, in particularly, was analyzed because it is known as an inhibitor of FSH synthesis and secretion, and therefore could possibly be termed as a direct marker of a decline of ovarian reserve. To relate the hormonal changes to the menstrual cycle, the serum progesterone was analyzed. FSH, LH and estradiol were analyzed by well-characterized immunoassay's and inhibin A and B were determined by specific two site enzyme immunometric assays.
Conclusion: The Results showed a statistically significant increase in serum FSH and LH and a accompanying decrease in estradiol and inhibins during the observation period before the FMP. Inhibin A showed a steady decline from at least four years before the final menstrual period until one year before menopause, whereas inhibin B had a shorter lasting decline from years three to year two before menopause. The continuous decline in inhibin A before the starting decline in inhibin B suggests that an increasing part of the cycle was anovulatory, a menstrual cycle that is characterized by varying degrees of menstrual intervals and the absence of ovulation and a luteal phase. The decrease in inhibin B and the rise in FSH together act as markers of ovarian aging. It was also observed that prior to one year before menopause neither inhibin A nor inhibin B were observed, the absence of these two peptide hormones also act as an indication of the oncoming menopause.
Critique: The paper "Inhibin A and B as markers of menopause" overall was well written and easy to understand. There were some gaps in the paper due to the lack of study in certain areas. For instances there are few reports on the changes in circulating immunoreactive inhibin concentrations measured longitudinally in the same subject during the menopausal transition period. However these discrepancies were taken into consideration. The study also produced results that confirmed and supported previous studies done in this same area, such as the experiment done by Burger et al, who documented a significant decrease in the levels of inhibins A and B before the final menstruating period. Overlie and colleagues also did an excellent job of explaining any possible reasons for the differences in the results such as why inhibin A and B levels were below detection limits in some women. Overall I felt the paper was well written and very easy to comprehend with very little need for further background information.
Future experiment:
Question:What is the relationship between inhibin and pre-puberty (menarche)?
Method: Collect serial samples during a prospective longitudinal trial and measure inhibin levels by a highly specific and sensitive two-site ELISAs.
Results: Inhibin levels will remain restricted before menarche, then rise as puberty proceeds.
Link to paper

 subunit is over expressed in second trimester placental tissue of pregnancy this is a indicator of fetal Down's syndrome
subunit is over expressed in second trimester placental tissue of pregnancy this is a indicator of fetal Down's syndrome